
過渡金屬硼酸鹽具有成本低、環保友好、電壓極化低和氧化還原電位合適等優點,是一種極具應用前景的新興材料。硼原子與氧原子的鍵合可以形成具有高電負性位點的陰離子(如BO33-、BO45-和BO69-),這些陰離子可以與過渡金屬陽離子鍵合,使得過渡金屬硼酸鹽具有不同的結構。盡管一些過渡金屬硼酸鹽表現出比相應的過渡金屬氧化物更優越的初始庫侖效率(ICE),但它們在基于碳酸酯電解液的鈉離子電池中具有較差的倍率性能和循環壽命,阻礙了其實際應用。
基于此,四川大學高分子科學與工程學院王延青特聘研究員課題組,在材料領域知名期刊Advanced Science上發表題為“Combustion activation induced solid-state synthesis for N, B co-doped carbon/zinc borate anode with a boosting of sodium storage performance”的研究論文。
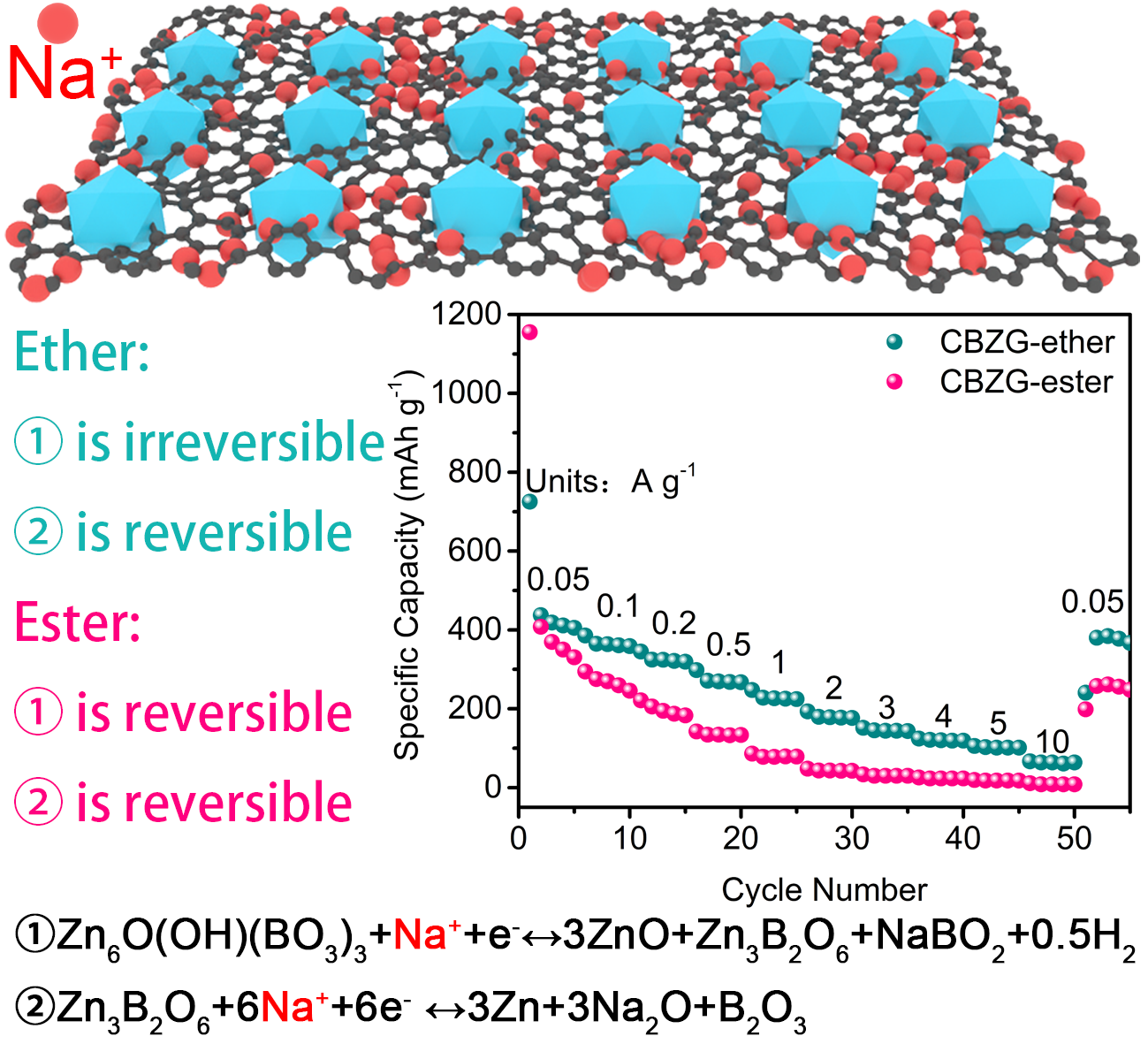
Figure 1. Na+ storage mechanism and rate performance of CBZG anode.
本文要點
要點一:基于插層誘導燃燒活化法合成了N、 B共摻雜碳/硼酸鋅(CBZG)
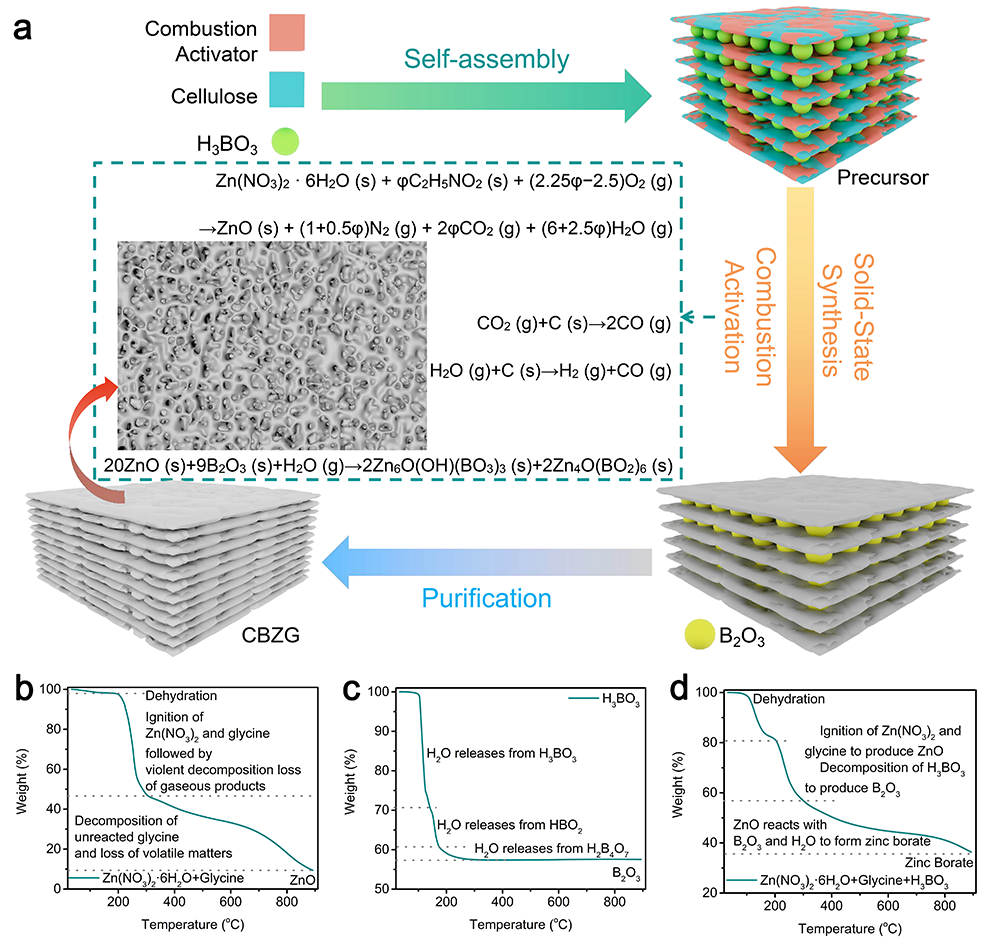
Figure 2. a) Illustration of the synthesis process for CBZG. TG curves of b) the mixture of Zn(NO3)2·6H2O and glycine, c) H3BO3, and d) the mixture of Zn(NO3)2·6H2O, glycine and H3BO3.
要點二: CBZG的形貌結構
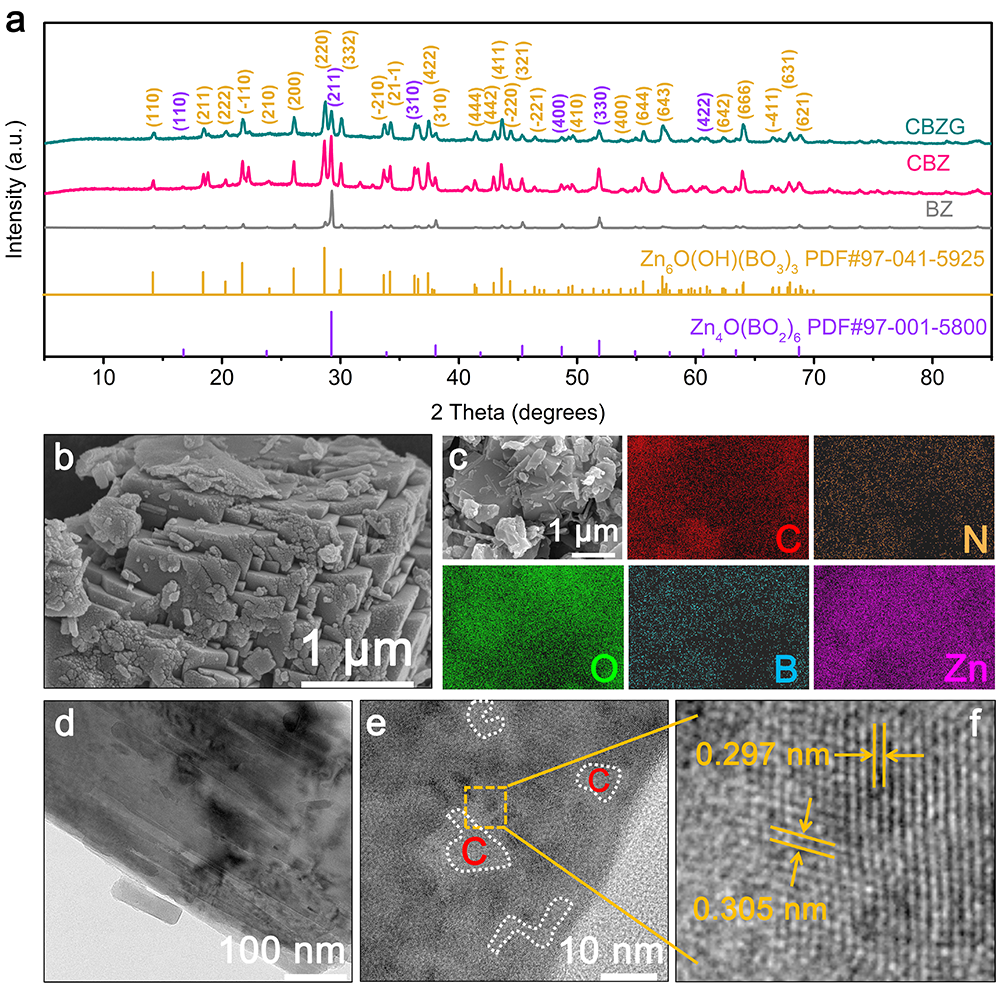
Figure 3. Structure and morphology of the obtained materials. a) XRD patterns of BZ, CBZ, and CBZG. b) SEM image of CBZG. c) Element mapping images of CBZG. d) TEM image of CBZG. e, f) High-resolution TEM images of CBZG.
要點三: CBZG在醚/酯基電解液中的鈉離子電池性能
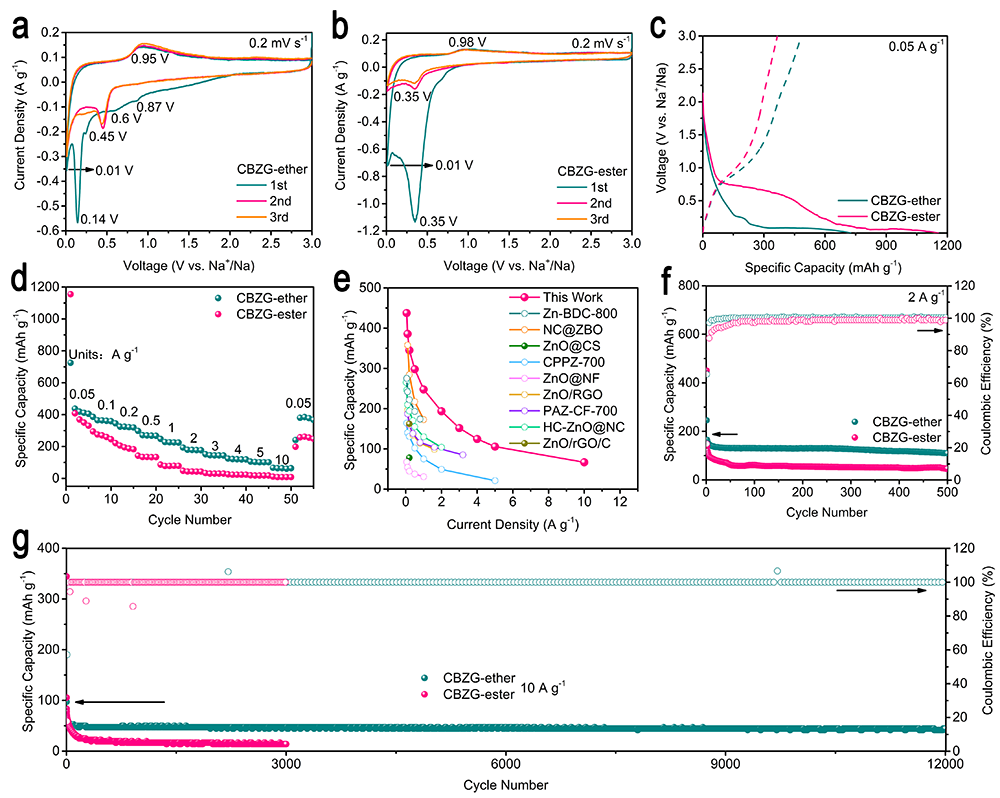
Figure 4. Na+ storage performance of CBZG as the half-cell anode in ether- or ester-based electrolyte. a, b) CV curves at 0.2 mV s-1. c) Discharge/charge profiles at 0.05 A g-1. d) Rate capability. e) Comparison between CBZG anode and previously reported SIB anodes in capacity. Cycling performance at f) 2 A g-1 and g) 10 A g-1.
要點四: CBZG在醚/酯基電解液中的儲鈉機理
Zn6O(OH)(BO3)3+Na++e-?3ZnO+Zn3B2O6+NaBO2+0.5H2①
Zn3B2O6+6Na++6e-?3Zn+3Na2O+B2O3②
反應①在醚基電解液中不可逆,而在酯基電解液中可逆。
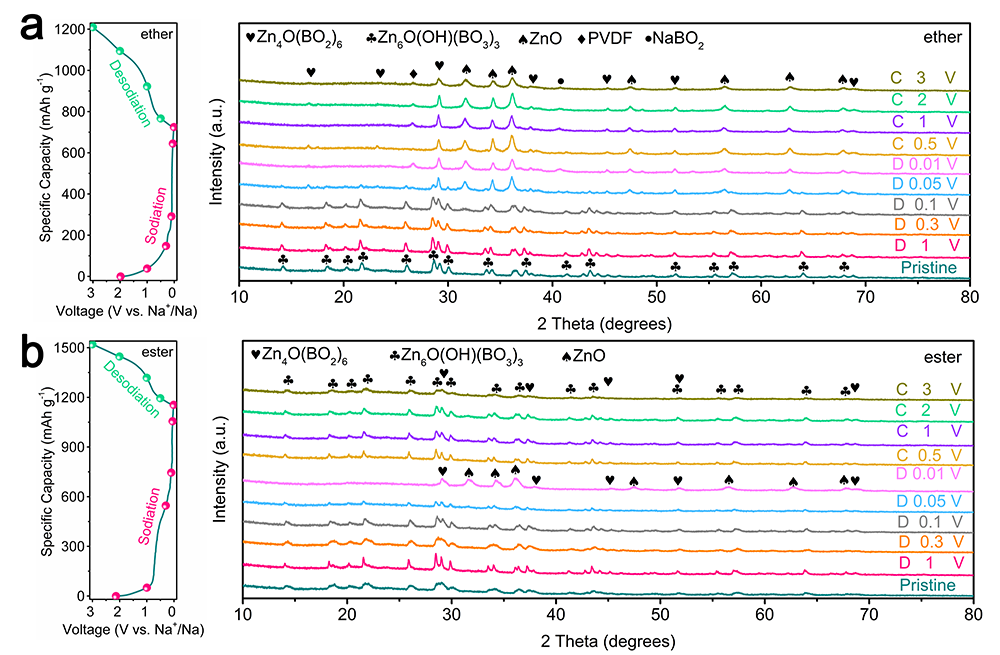
Figure 5. Na+ storage process analysis of CBZG as the half-cell anode in ether- or ester-based electrolyte. Discharge/charge profiles at 0.05 A g-1 and ex situ XRD patterns under various stages in a) ether-based electrolyte and b) ester-based electrolyte.
要點五: CBZG在醚/酯基電解液中的動力學過程
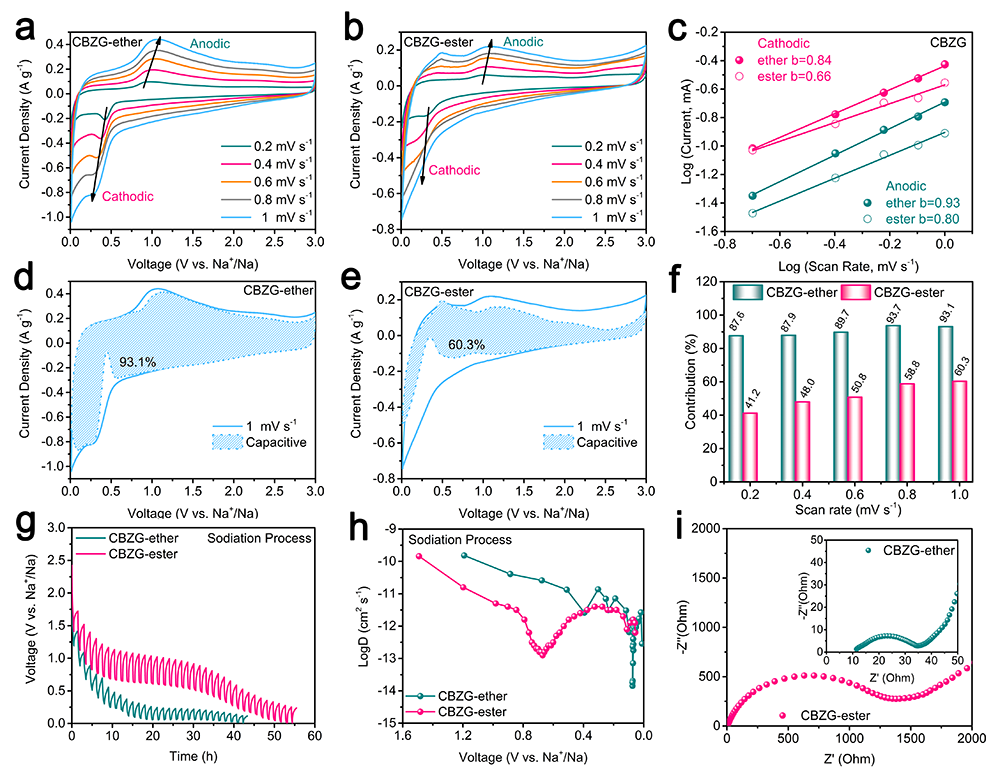
Figure 6. Kinetics analysis of Na+ storage of CBZG anode in ether- or ester-based electrolyte. a, b) CV curves at different scan rates. c) b-value calculation. d, e) Capacitive contributions at 1.0 mV s-1. f) Capacitive contributions at different scan rates. g) GITT potential profiles with a pulse current of 0.05 A g-1 for 0.5 h, followed by a 1.0 h relaxation process. h) Na+ diffusion coefficients calculated from the GITT potential profiles for the discharge process. i) Nyquist plots.
文章鏈接:Combustion activation induced solid-state synthesis for N, B co-doped carbon/zinc borate anode with a boosting of sodium storage performance
DOI:10.1002/advs.202207751
http://doi.org/10.1002/advs.202207751
通訊作者簡介
王延青博士,男,四川大學特聘研究員,四川省“海外高層次人才引進計劃”特聘專家,國家制革技術研究推廣中心特聘專家,四川省專家服務團專家,日本政府高端引進外國人(日本高度人才1號)。入選四川大學“雙百人才工程”計劃(2019-2023),日本學術振興會(JSPS)外國人特別研究員(2015-2017)。2019年加入四川大學高分子科學與工程學院高材系獨立開展研究工作,成立先進碳與能源材料應用研究室。主要從事超長碳納米管的單分散原理、碳基材料的設計制備及其在能源、環境相關領域的應用研究,主要包括:超長碳納米管在非/弱極性有機體系的分散研究、新型高倍率快充鋰電池導電劑、低溫鋰電池負極、鈉電池硬碳負極、電磁屏蔽/吸波材料、超級電容器、碳基導熱/散熱材料、柔性顯示材料、先進高分子功能材料等,在Advanced Science(2篇),Carbon(8篇),Chemical Engineering Journal,Small,J Mater Chem A,Energy Storage Materials等高水平學術期刊上發表論文40余篇。研究成果獲得了山東省科技進步一等獎、國家優秀自費留學生獎學金、中國專利優秀獎、山東省專利獎、四川省特聘專家、JSPS外國青年學者研究獎勵、北海道大學私費外國人留學生特待制度、四川大學優秀科技人才獎、鹽都特聘專家等。
