研究背景
在碳基材料中,拓撲缺陷是不可避免的存在,但這些缺陷的固有電催化活性和機制尚未完全探索。綠色氫作為環保的零碳能源,在未來氫能系統中起著關鍵作用,主要通過使用可再生能源(如太陽能和風能)進行水電解來生產。然而,目前綠色氫的經濟可行性低于灰氫等其他類型,且貴金屬催化劑(如Pt/C、IrO?、RuO?)在水電解中仍不可或缺。特別是,對于氫析出反應(HER),昂貴的Pt仍然占據催化性能的頂峰。因此,研究如何使用更便宜的貴金屬(如Ru)并探索其催化機制,對于降低水電解成本具有重要意義。
工作內容
通過密度泛函理論(DFT)計算,預測了富含五邊形環的碳(PRC)結構中五邊形環的電化學反應性。發現五邊形拓撲缺陷能夠優化碳基體的電子性質,如降低帶隙能量、優化p帶中心和提高電子再分布程度,從而調節關鍵中間體的吸附。通過堿刻蝕技術處理富勒烯材料(C60),形成富含五邊形環的碳納米材料(PRC)。使用濕化學法和熱還原法將Ru物種錨定在PRC上,形成Ru@PRC催化劑。利用光譜學和電子顯微鏡技術,揭示Ru@PRC中五邊形缺陷的關鍵作用,以及C原子和Ru原子之間的p-d軌道雜化效應。研究表明,C-p軌道和Ru-d軌道之間的強雜化調節了Ru@PRC結構的電子狀態,增強了其HER活性。測試了Ru@PRC催化劑在不同pH介質中的HER活性,特別是在堿性條件下表現出優異的性能。Ru@PRC在電流密度為10 mA cm?2時,過電位僅為28 mV,遠低于Pt/C催化劑。
工作創新點
1.揭示了五邊形缺陷對HER的積極作用:首次通過實驗和理論相結合的方法,證明了五邊形缺陷可以作為HER的活性位點,顯著提高碳基材料的HER活性。
2.p-d軌道雜化效應:提出了C原子(五邊形環中)和Ru原子之間的p-d軌道雜化效應,這種效應促進了電子從Ru簇向五邊形環的快速轉移,減弱了Ru與氫中間體的結合強度,從而增強了HER活性。
3.高效催化劑的制備:成功制備了Ru@PRC催化劑,該催化劑在HER中表現出優異的催化活性和穩定性,特別是在堿性條件下,其性能顯著優于商業Pt/C催化劑。
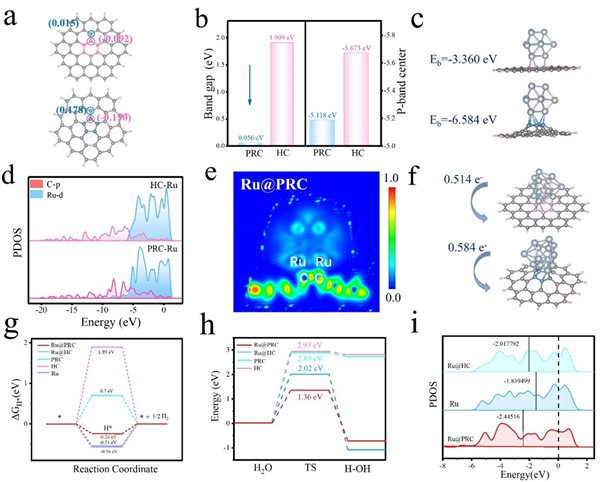
Figure 1. (a) Charge densities of PRC and HC. (b) Band gap and calculated p-band center of PRC and HC. (c) Geometric optimization results and binding energies of Ru@HC and Ru@PRC. (d) PDOS of C, Ru for Ru@PRC and Ru@HC. (e) ELF analyses of Ru@PRC. (f) Bader charge transfer on Ru@HC and Ru@PRC models. (g) HER Gibbs free energy diagrams of Ru@PRC, Ru@HC, PRC, HC and Ru models. (h) Water-dissociation pathways of Ru@PRC, Ru@HC, PRC, HC models. (i) d-band center analyses of Ru@HC, Ru and Ru@PRC.
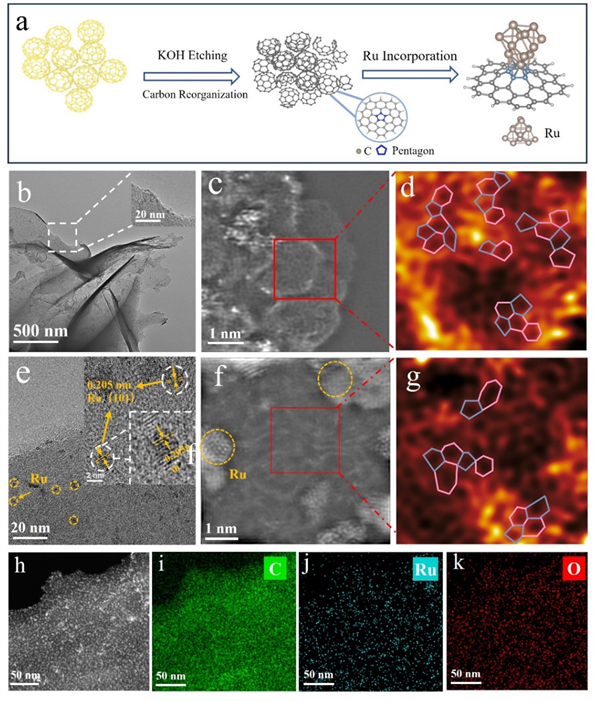
Figure 2. (a) Schematic of the synthesis process for Ru@PRC. (b) TEM images of PRC. (c) Ac-STEM image of PRC. (d) Filtered image of PRC. (e) TEM image of Ru@PRC. (f) Ac-STEM image Ru@PRC. (g) Filtered image of Ru@PRC. (h-k) Elemental mapping of Ru@PRC.
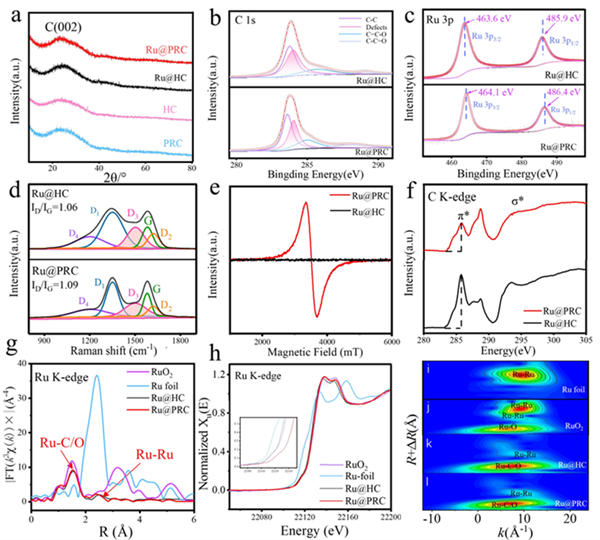
Figure 3. (a) XRD patterns of Ru@PRC, Ru@HC, PRC and HC. (b) C 1s and (c) Ru 3p fitting results of Ru@HC and Ru@PRC. (d) Raman spectra and (e) EPR results of Ru@HC and Ru@PRC. (f) C K-edge NEXAFS spectra of Ru@PRC and Ru@HC. (g) EXAFS and (h) XANES spectra and (i-l) wavelet transform (WT-) analyses of RuO2, Ru foil, Ru@HC and Ru@PRC.
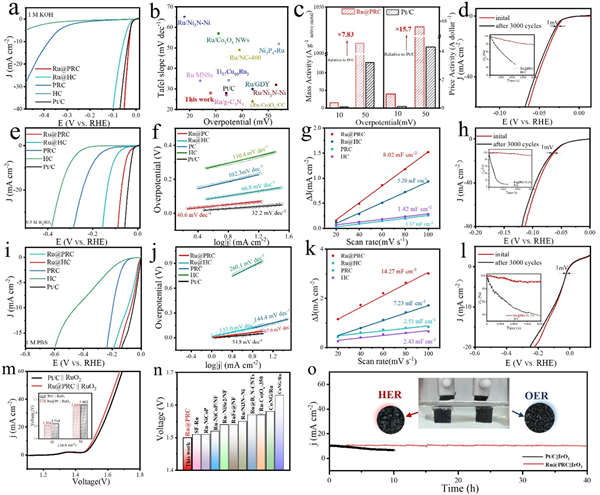
Figure 4. (a) Polarization curves (with iR-correction) of Ru@PRC, Ru@HC, PRC, HC and Pt/C catalysts in 1 M KOH solution. (b) Comparison of catalytic performance of Ru@PRC with other reported alkaline HER catalysts. (c) Mass activity and price activity at the overpotentials of 10 and 50 mV for Ru@PRC and Pt/C catalysts. (d) Stability test of Ru@PRC in alkaline media. (e) Polarization curves (with iR-correction) of Ru@PRC, Ru@HC, PRC, HC and Pt/C catalysts in 0.5 M H2SO4 solution. (f) Relevant Tafel slopes. (g) Cdl calculation of Ru@PRC, Ru@HC, PRC, HC, and Pt/C in acidic media. (h) Stability test of Ru@PRC in acidic media. (i) Polarization curves (with iR-correction) of Ru@PRC, Ru@HC, PRC, HC and Pt/C catalysts in 1 M PBS solution. (j) Relevant Tafel slopes. (k) Cdl calculation of Ru@PRC, Ru@HC, PRC, HC and Pt/C in neutral media. (l) Stability test of Ru@PRC in neutral media. (m) Polarization curves of overall water splitting performance of Ru@PRC || RuO2 couple and Pt/C || RuO2 couple in alkaline media. (n) Comparison of overall water splitting performance of Ru@PRC with other reported alkaline HER catalysts. (o) durability test of Ru@PRC || IrO2 and commercial Pt/C|| IrO2.
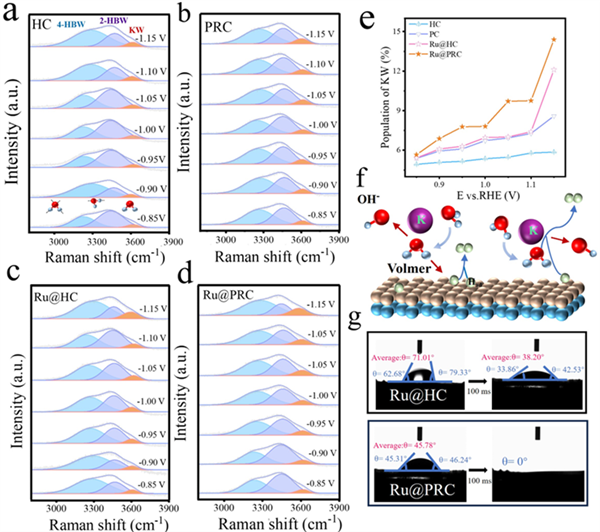
Figure 5. In situ Raman spectra of interfacial water on (a)HC, (b) PRC, (c) Ru@HC and (d) Ru@PRC in 1M KOH solutions (E versus RHE). (e) The population of KW from in situ Raman spectra. (f) Schematic showing interfacial water dissociation on Ru@PRC. (g) Contact angles of Ru@HC and Ru@PRC using the KOH electrolyte.
原文信息
Lei Gong, Fanjie Xia, Jiawei Zhu, Xueqin Mu, Ding Chen, Hongyu Zhao, Lei Chen, Shichun Mu, Hydrogen Evolution Reactivity of Pentagonal Carbon Rings and p-d Orbital Hybridization Effect with Ru, Angew. Chem. Int. Ed. 2024, e202411125.
https://doi.org/10.1002/anie.202411125